| View previous topic :: View next topic |
| Author |
Message |
Roger Warin
Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 14:21 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 14:21 Post subject: Re: Libyan Desert Glass Structure |
|
|
Hi,
I intend to present my ideas to you. Just a little more patience
It is still complex to describe the LDG in a few words.
At least the photos will surprise you.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 14:47 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 14:47 Post subject: Re: Libyan Desert Glass Structure |
|
|
Hi LDG lovers - FMF LDG
At the risk of sounding like an iconoclast, I'm a chemist who became one because he loved minerals. My experience as a researcher-teacher can tell you that I'm not afraid of synthesizing molecules on a bench, nor of spectrometers, since I ended my career specializing in NMR (liquids and solids-MAS).
But I do like simple ideas, and that's when I get things wrong.
I've been collecting thin sections of meteorites since the '90s. Being very close to Zelimir Gabelica, I visited all the shows in Ensisheim (France).
So it's been a long time since I've held this famous desert item in my fingers.
I've given it a lot of thought, and at first I was wrong to subscribe to Norbert Brugge's (Germany) theories on the terrestrial origin of Libyan glass. His arguments seemed very plausible and respectable, especially as no coherent thesis had been published.
The crux of the matter for me, as a chemist and amateur geologist, was that silica glass is 98-99% SiO2.
I really understood the problem when Marcos Campo Venuti gave me the answer on the origin of Pele's tears, on this Jordi forum.
So here's my point of view, and I'm sorry if I come across as an iconoclast.
I only ask a question that I believe to be original, like some of my photos (taken in January 2013) that remain unpublished.
And of course the discussion is open.
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 14:50 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 14:50 Post subject: Re: Libyan Desert Glass Structure |
|
|
LDG STORY
Around 26 million years ago, a cataclysm occurred in Egypt, in a place that today belongs to the Libyan Desert. The presence of Libyan Desert Glass (LDG) bears witness to this phenomenon. This LDG glass was used as a tool during the Pleistocene.
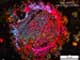
This glassy phase can be gem-quality, but this is very rare. The photo shows a strictly transparent specimen containing cristobalite inclusions.
These photos actually show crystalline spherules, not single crystals. They were formed in an almost pure silica gel.
The origin of this gel explains the cause of the phenomenon.
In response to my question, a director of the French CNRS told me that the fall of a celestial body could have occurred on a mass of diatoms (whose shell is made of almost pure silica).
Objection : the probability of such an event occurring is very low.
In fact, there is a similarity (without witnesses) with the Tunguska blast (Siberia - 1908) caused by the partial fall and high-altitude explosion of a comet.
LDG glass is not impactite or tectite, but an “exudate” of a terrestrial rock, a sandstone rock from this African territory, which was not a sandy desert, but a kind of savannah. These silicate rocks have lost SiO2, which in these Dantean conditions is soluble in water (yielding numerous complex orthosilicic and polysilicic acids – also tectosilicates)). These silica gels are currently destined for major industrial applications.
| Mineral: | Libyan Desert Glass |
| Description: |
|
| Viewed: |
42701 Time(s) |

|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 14:55 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 14:55 Post subject: Re: Libyan Desert Glass Structure |
|
|
The LDG precursor gel obviously contains traces of the signature of celestial bodies (astrophysicists' famous dark dust). This dark matter is comparable to the very primitive cold carbonaceous chondrites of the Ivuna (CI) or Mighei (CM2) type.
These space-origin impurities include compounds similar to those found in CAI's and, as the best signature, space-origin iridium (~ 150 ppm Ir) (crustal abundance of Ir on Earth = 0.001 ppm - the lowest value of all natural elements).
Striations in LDG (Schlieren) are correlated with LDG viscosity.
Silica gel (not dehydrated) is an amorphous phase with siloxane bridges created by condensation of two intermediate silanol groups with the loss of one water molecule.
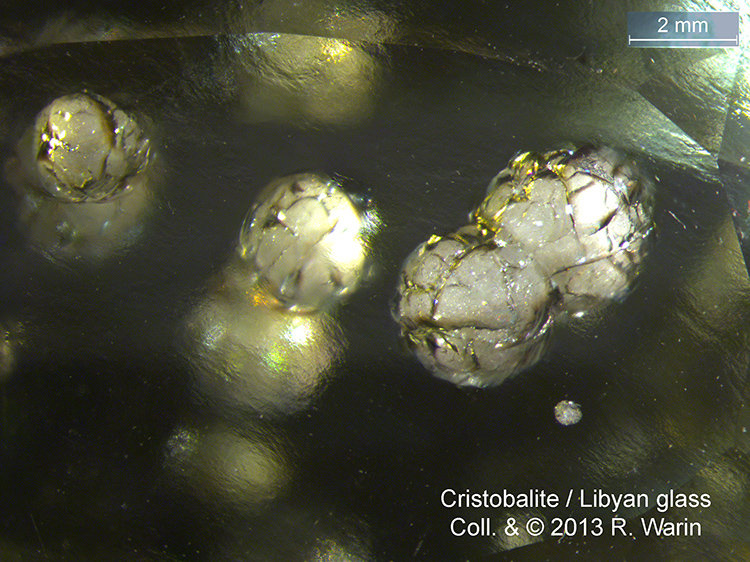
In fact, this amorphous “tectosilicate” phase sometimes gives up crystalline anhydrous SiO2 in the structure of high-temperature, high-pressure amorphous phases, such as cristobalite (β- Cristobalite is a material that is NOT an approved mineral species (IMA). Also Tridymite, Lechatelierite (amorphous) in these high temperature conditions between 870 and 1470°C.
| Mineral: | Cristobalite |
| Description: |
|
| Viewed: |
42698 Time(s) |
|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 14:58 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 14:58 Post subject: Re: Libyan Desert Glass Structure |
|
|
I was surprised when I found on my photo the Maltese cross that I had already shown you in my photos of luxulianite (UK).
Indeed, spherulites have particular optical properties. Thus, observed under an optical microscope between a crossed polarizer and analyzer, they often present an extinction in the shape of a Maltese cross, along the axes of the polarizer and the analyzer.
The optical properties of spherulites result from the dielectric nature of the phases, the anisotropic character of the crystals, and the particular organization of these crystals within the spherulite. This texture induces an extinction in the shape of a Maltese cross whose position is constant and independent of the rotation of the microscope stage.
| Description: |
|
| Viewed: |
42702 Time(s) |

|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 15:00 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 15:00 Post subject: Re: Libyan Desert Glass Structure |
|
|
Note that cristobalite spherulites with semi-crystalline phases are similar to those observed in crystallography of (bio)polymers.
| Description: |
|
| Viewed: |
42706 Time(s) |
|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 15:01 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 15:01 Post subject: Re: Libyan Desert Glass Structure |
|
|
OTHER CURIOSITIES: - SCHLIEREN – BUBBLES - SPACE MATERIAL
As for Schlieren, they result from an optical phenomenon highlighting the local contractions and decompressions of the gel in its flow which does not seem to have been turbulent during a slow cooling.
This German term has become a general characteristic of glasses; it means "striations". These Schlieren materialize the lines of flux by deflecting the light because of the existence of a gradient of refractive indices. These differences in index are correlated with the gradient of the flux density. This pattern of curved shadows follows the regions of low density (expansion) and high density (compression).
Other inclusions are numerous to the point that truly clear specimens are very rare. Chains of microbubbles or bubbles, particularly of cristobalite, are also found. Bubbles are small cavities created during the dehydration of silica gel.
There are also chains of microbubbles or bubbles. Bubbles are small cavities created during the dehydration of silica gel.
| Description: |
|
| Viewed: |
42692 Time(s) |

|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Jan 14, 2025 15:03 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 15:03 Post subject: Re: Libyan Desert Glass Structure |
|
|
Some bubbles and Sclieren in LDG. © R. Warin.
BLACK VEILS :
This phase presents traces of siderophile elements including iridium, osmium, platinoids, which form black veils during drying.
In fact, we find space dust there, many of which are found in primitive chondrites.
This means that the minerals found in the CAI’s are also found in Libyan glass. The origin of these compounds is truly spatial.
This phase presents traces of siderophile elements including iridium, osmium, platinoids, which appear during the drying of the gel.
The black bands contain carbon (the black dust of astrophysicists), traces of siderophile elements such as Co, Ni, Ir, Re, Os which are attributed by most authors including Koeberl as being traces proving the fall of a chondrite. Koeberl did, however, recognize compositional differences between the surrounding desert sands and the Libyan glasses. In fact, I am more likely to think of a cometary nucleus or the material that fouls comet ice. Incidentally, the ice is in a special polymorphic phase at -220°C.
Analysis of the dark streaks in the LDG reveals high abundances of Al, Ti, Mn, Cr, Fe, and Ni, as well as a pronounced correlation between the abundances of Cr, Mn, Fe, and Ni. Since the Fe/Ni, Mn/Ni, and Cr/Ni ratios are all clearly nonchondritic, the source of this material is most likely extraterrestrial, a comet.
CONCLUSION :
The existence of cristobalite in LDG glass proves that this silica phase underwent significant heating, of the order of 1800 °C. This argument simply specifies the temperatures that were reached during the extraction of pure silica SiO2 that formed the silica gel that became LDG.
It would seem that I am the only one to compare this origin of LDG to the partial fall of the Tunguska Bolide (explosion of a comet at an altitude of 5-10 km and fall of a solid debris from this comet that dug Lake Ceko – June 30, 1908). Small cavities, without craters, were found at the epicenter, with iridium, of course.
In Egypt, it was the explosion of another comet that provided the solvent needed to extract SiO2 from local silicate rocks.
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 234
Location: Sevilla



|
 Posted: Jan 14, 2025 17:06 Post subject: Re: Libyan Desert Glass Structure Posted: Jan 14, 2025 17:06 Post subject: Re: Libyan Desert Glass Structure |
|
|
Hi Roger, beautiful photos and interesting discussion. I am always happy to discuss with you. I would like to clarify some points on which I have studied for a long time especially on the origin of spherulites. I am a volcanologist and the problem of natural glasses has always touched me closely.
1- Who did you get the term "exudate" from? It sounds similar to what I had defined as partial melting. However, the term exudate in geology has another meaning, so it does not seem appropriate in this context. The concept is that during the explosion of the comet the large amount of water allows the partial melting of a highly silicate component. Indeed, this fraction exudes from the rocks. In fact, to have a complete melting it lacks the time. If I completely melt the mantle I have a peridotite, with a partial melting I have a basalt, but with a very limited partial melting I can also have a rhyolite. There are rhyolites associated with ocean ridges. It is not necessary to invoke a siliceous substrate (diatomeite) because any rock can be ok to extract the LDG by means of partial melting.
2- Silica gel is not stable at temperatures above 300 C. What you call gel I believe is a high temperature melt. It is not magma, it is not lava, but it is certainly not a gel. In the models that seemed more plausible to me I had read about sublimation, so it would correctly be a vapor, the result of sublimation. When the steam condenses then we have glass. For this reason we are not in a context of high pressure, but only high temperature. Because we are in open space. High pressure minerals are found at the bottom of a crater, but not in a melt that forms in air.
3- I do not see analogies with Tunguska because in that event there is no glass or any other fragment of extraterrestrial origin. So even if it were a comet, and I am not sure because we have never found the smoking gun, it would be a small comet that was unable to form glass. So your analogy is a bit risky.
4- Where I disagree with you and many scholars is on the origin of spherulites, the subject of a work of mine in preparation, but based on a hypothesis already proposed many years ago.
Spherulites are the main theme of many misinterpretations in geology and especially in the study of obsidians and the genesis of thunder eggs. Spherulites form at low temperatures and are not due to crystallization in a fluid. In a silicate magma if something crystallizes phenocrysts of a single mineral are formed, not polycrystalline spherules that include different minerals. Furthermore, the composition of spherulites includes amorphous silica and cristobalite, two minerals that are incompatible with each other and both incompatible with a rhyolitic magma. They form at very different temperatures, one at room temperature and the other at >1470 C. Cristobalite is nothing more than opal as is that of snowflake obsidian. Finally we saw that obsidians are aphyric precisely because they have a Tg that is too high and do not have time to crystallize. Some authors believe that crystallization occurs below the Tg but at temperatures maintained high for years. Obsidian remains fluid for minutes, not years. Even in tektites and in LDG that I consider a tektite, cooling is very rapid. We are in a drop of glass that is falling. No fragment has been found with evidence of deformations from impact with the ground so they fell already cold. Basaltic volcanic bombs have the shape of cow dung because they fall still molten. Schlieren or fluid structures must form very quickly. As well as the few bubbles that indicate an albeit brief degassing.
Below the Tg, obsidian is already solid and therefore cannot crystallize. What is the difference between a high temperature spherulite (HTCD, high temperature crystallization domains by some authors) and a phenocryst? Why should we have high nucleation at high temperature? The HTCD model simply does not work. All experimental works on spherulites shows pictures of groups of crystals aggregated around a nucleus, therefore phenocryst aggregates, but not microcrystalline spherical structures and therefore not spherulites.
Spherulites form in any ancient glass and are well known in the glass of the Murano dump and in many medieval stained glass windows. Any archaeologist is familiar with these structures and they call it pitting. The Alkali Silica Reaction dissolves glass at room temperature and transforms it into a hydrated silica gel (this is a gel). This reaction starts from a point, often an air bubble or a small crystal of feldspar (alkalis help the reaction because at pH>9 the solubility of silica becomes very high) and creates a spherical cavity. Obsidian is porous for water which has a small molecule, but the molecules of silica polymers are large and cannot escape. So the water enters, but the silica gel remains trapped. However if the ASR is very intense we can produce silica monomers which are molecules small enough to escape. This gel then recrystallizes inside a spherical space at room temperature and various poorly crystalline minerals are formed.
Your beautiful photos show spherulites with obvious desiccation cracks. The supposed Maltese cross honestly does not convince me. They have a septarian type crack model so they indicate a volume decreasing. In fact the ASR is a reaction that consumes water and the result is a hydrated gel. When this gel dries, cracks form.
will continue ...
|
|
| Back to top |
|
 |
Craig Hagstrom
Joined: 10 Jan 2025
Posts: 26


|
 Posted: Mar 14, 2025 12:47 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 14, 2025 12:47 Post subject: Re: Libyan Desert Glass Structure |
|
|
Uploaded a new version. This has some improved images - getting better with that microscope. I laid out three evidentiary tests for my thesis, and added some graphic aids. The coolest addition is a specimen with embedded leaf fragments, and if that sounds unlikely to you, it did to me as well.
I put it on Academia.edu, looks like a better place for people to find it.
https://www.academia.edu/128186762/Libyan_Desert_Glass_Structure
(link normalized by FMF)
| Description: |
|
| Viewed: |
40709 Time(s) |

|
| Description: |
|
| Viewed: |
40698 Time(s) |

|
|
|
| Back to top |
|
 |
Roger Warin

Joined: 23 Jan 2013
Posts: 1240



|
 Posted: Mar 17, 2025 03:46 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 17, 2025 03:46 Post subject: Re: Libyan Desert Glass Structure |
|
|
Hello Marco,
Today, I am going to shock you: quartz is a mineral species that almost never exists as such. This was already the despair of “des Cloizeau” (famous French mineralogist (1817-1897) honored by descloysite). With its framework-like texture, the polymorphic phase changes of quartz do not require much energy to give hand changes in the Dauphiné-twin or Brazilian-twin.
This framework is made up of Si-O bonds in 3D. Most quartz crystals have internal twinning (Dauphiné or Brazilian twins), which means that these crystals have lost their piezoelectric properties, for example, forcing manufacturers to carry out controlled syntheses of quartz.
Regarding the LDG, I have seen many photos, but never those of a thin section (TS). Not long ago, I ordered the cutting of such TSs, in fact of the black trails that are the residue of a comet impact. I understand that scientists have never uttered the word comet, because they had no bibliographic references to say so.
My age allows impertinence, while maintaining great respect for the FMForum:
to my knowledge, two cometary nuclei have recently struck us in the recent period : on the one hand, 28 million years ago in southern Egypt, a region that I think was a savannah. It was not a desert and there was no sand, but rather sandstone typical of this part of North Africa.
The second comet is the Tunguska comet at the beginning of the 20th century. This was more of a tangential flyby of the fireball over more than 1000 km before its explosion at high altitude (5-10 km) at the epicenter.
I can personally send you a long PDF that supports my thesis, as I believe this forum cannot accept it.
One point never emphasized by astrophysicists: water can become an explosive above 800°C and totally so at 5000°C (hydrogen + oxygen). Lavoisier, one of the founders of modern chemistry, told me so!
I know that we no longer consult old references! The Moscow fire brigade was unaware of this when they used too little water to extinguish the burning theater a short time ago.
I used the term exudate because I am close to Nature (I was even a beekeeper from around 1962 to 2020). An organic sap (like amber) can be exuded from trees. A participant in this forum opened my eyes when I presented the tears of the Hawaiian goddess. It was you, Marco. I thank you for that.
You opened my eyes when we were talking about Pele's hair. LDG has always been an exception for me, as a chemist. This glass is composed of more than 98% SiO2. This is an exception among natural glasses.
Where did this pure silica come from? No sand (except diatom shells, because these small organisms extracted the silica from seawater) has this composition. Sand has a powdery texture of rocks that were made of feldspars and other silicates. To reach a level of 98% SiO2, it is necessary to extract the silica from the silicate crystals, using heat and water. This water has nothing in common with municipal water. Its composition can no longer be located on a phase diagram, because the state of equilibrium of the phases is never reached in these Dantesque conditions and this notion of a diagram no longer exists.
Water is not a gentle solvent; it is a formidable reagent with regard to silicates.
H2O breaks the bonds of silicate minerals to form aggregates rich in silanol (Si-OH) functions that separate from the bedrock. As this chemical reaction progresses, the water disappears, not to give a gas but to generate a silica gel that is more or less hydrated, i.e. rich in silanol functions + incorporated H2O molecules.
The future of this SiO2-rich phase is uncertain.
We will obtain chalcedony and other poorly defined mineral silica phases.
It is this silica gel that I call rock exudate. Under milder and more controlled conditions in alpine fissures, beautiful, clear, idiomorphic quartz crystals are obtained. Despite this, they are very often twinned and often retain a soul (Faden quartz).
The fall of a comet induces even higher temperatures and pressures. As a result, the gods of Olympus gave us this wonderful Libyan glass, which can be a gemstone, without the presence of crystalline structures, except for a few secondary polymorphic crystallizations of SiO2 at high temperature (cristobalite, etc).
Schlieren are interesting because they remain evidence of these flows, the study of which has made it possible to understand the cooling of this material.
In addition, this LDG has brought with it that black space dust that all astrophysicists talk about without daring to define it (no interactions with spectrometers). This proves its distant origin, which was certainly not from a rocky asteroid, even a primitive and carbonaceous one, but a comet.
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 234
Location: Sevilla



|
 Posted: Mar 17, 2025 15:29 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 17, 2025 15:29 Post subject: Re: Libyan Desert Glass Structure |
|
|
Hello Roger,
I was in the Tunguska Italian team of 1991 with the physicists from Bologna and I was the only geologist, in charge of distinguishing the particles of volcanic origin from those potentially of extraterrestrial origin found on the tree trunks felled by the explosion. There are numerous important eruptions that have brought suspended ash in the atmosphere in the years immediately preceding the Tunguska event. Even then we talked about a comet, but how to prove it? I read that now Russian scientists say they found a block of ice belonging to a comet on the bottom of the Suslov crater.
However, I am a volcanologist and I arrived at the study of tektite glasses starting from the study of volcanic glasses. Analyzing the properties of high silica glasses it turns out that the classic statement that a lava that cools rapidly produces obsidian is false. What is important is only the absence of water (flux) that raises the glass transition Temperature (Tg), the temperature at which the glass becomes rigid and therefore stops crystallizing. So obsidian is the result of the eruption of an anhydrous magma.
A hydrated rhyolitic magma at 900C has a Tg of 400C so during the cooling from 900 to 400 it has time to crystallize and form a rhyolitic rock. The same magma when anhydrous has a Tg of 800C and so when it cools from 900C to 800C it becomes soon rigid and cannot crystallize, forming an obsidian.
The complicated part is that to melt a rock you need water. For example in subduction zones magma chambers form because the subduction transports water to depths and here the water acts as a flux. So you have to find a mechanism to dehydrate this magma and form obsidian.
Anhydrous glasses are only obsidians and tektites and so in both cases you have to understand how this water is lost. So to understand tektites you have to first understand obsidians well.
The mechanism of sublimation through a partial melting of terrestrial rocks by a comet reproduces a mechanism similar to that of obsidian. First we have a high water content that acts as a flux and favors partial melting and then overheating of the melt that allows dehydration. With this reasoning I can define a tektite as the result of the impact of a comet without "evidences" that demonstrates it. I hypothesized this process in the 80s before any scholars suggested it. Today we know that all tektites including the LDG are associated with comets. Unfortunately modern scientific journals demand numerical data and do not consider the simple use of logic as research.
This model for the genesis of tektites also implies that melting cannot be in situ as suggested by Craig in his Dune Glass model, because in that case we would have a hydrated glass as happens in fulgurites.
|
|
| Back to top |
|
 |
Bob Morgan
Joined: 18 Jan 2018
Posts: 251
Location: Savannah, Georgia



|
 Posted: Mar 17, 2025 16:50 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 17, 2025 16:50 Post subject: Re: Libyan Desert Glass Structure |
|
|
This discussion is far beyond my pay grade but thanks to you all understandable.
Perhaps you might be interested in the latest issue of Elements magazine. It is oriented around the role of water in various newly reported processes of mineral nucleation. Without water the energy barrier is too great to form crystal nuclei. Hence, as per this discussion, glass.
|
|
| Back to top |
|
 |
alfredo
Site Admin

Joined: 30 Jan 2008
Posts: 1013



|
 Posted: Mar 18, 2025 03:10 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 18, 2025 03:10 Post subject: Re: Libyan Desert Glass Structure |
|
|
Marco, thank you for this very interesting explanation.
I'm wondering what you mean by "anhydrous" magma yielding obsidian, because obsidian does contain a little bit of water. One common test for differentiating obsidian from tektite glasses is to heat a thin fragment in a gas flame. Obsidian will expand into frothy glass at its melting point because of the steam generated, whereas tektites will not, so I suppose "anhydrous" is a relative expression here and does not mean that obsidians have zero water content.
|
|
| Back to top |
|
 |
marco campos-venuti

Joined: 09 Apr 2014
Posts: 234
Location: Sevilla



|
 Posted: Mar 18, 2025 03:10 Post subject: Re: Libyan Desert Glass Structure Posted: Mar 18, 2025 03:10 Post subject: Re: Libyan Desert Glass Structure |
|
|
Yes Alfredo,
Obsidians often have a water content below 0.1%, compared to at least about 2% of a rhyolite which can be up to 7%. Tektites are almost anhydrous, <0.02%. The LDG is a somewhat anomalous tektite for having a water content of 0.64%. In fact, it is the only tektite that contains spherulites which are produced precisely by the Alkali Silica Reaction which needs water to occur. I suppose this difference is due to different parameters of the impact such as size of the comet, angle of impact, etc.
Generally, eruptions that produce obsidian lava flows begin with a pumice cone. In fact, the magma chamber accumulates gases in the upper portion that produce pumices. Therefore, obsidian represents the lower portion of the magma chamber and of course the terminal phase of the eruption. Similarly, in tektites we have in the proximal areas glass in blocks of larger dimensions and with a greater content of bubbles (Muong Nong-type of Australasian tektites and LDG). I do not know of models that explain this internal variation, but it undoubtedly has to do with a less strong degassing as in pumice.
|
|
| Back to top |
|
 |
|





